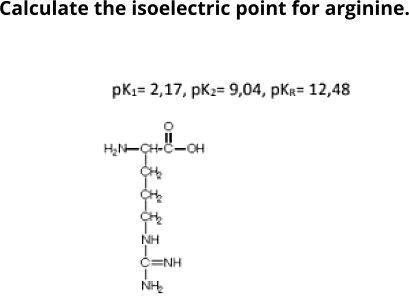
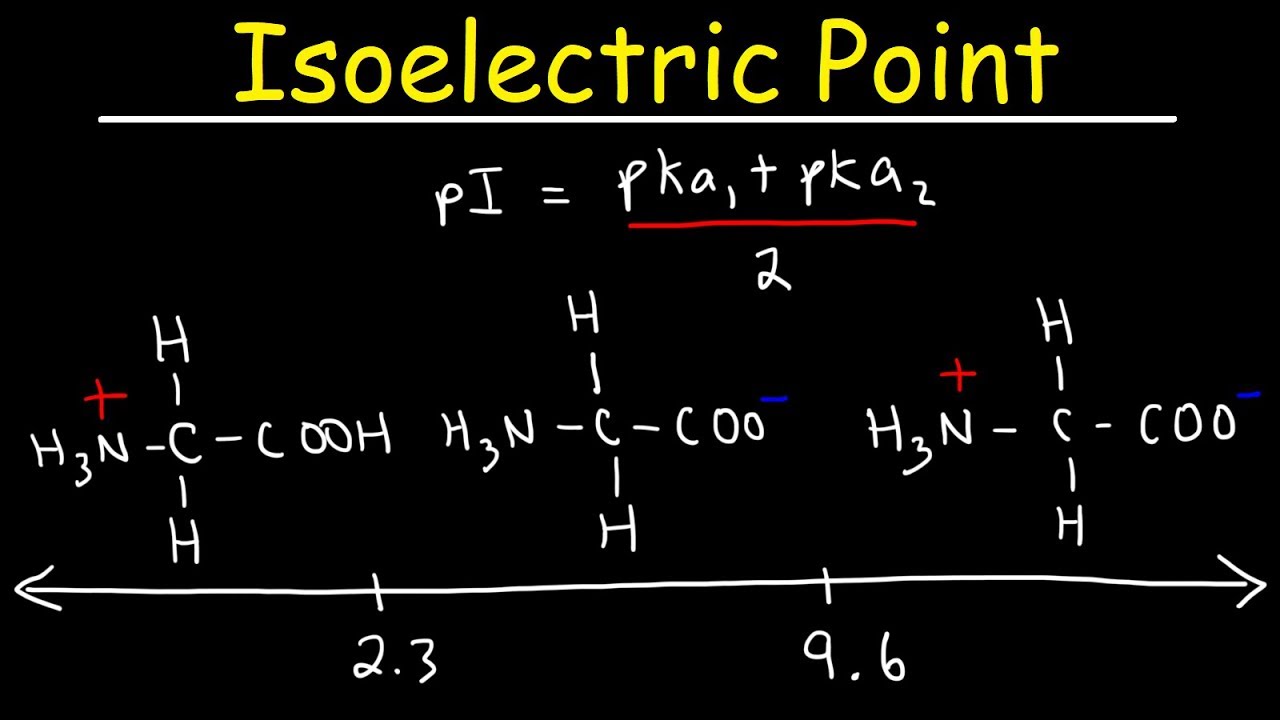
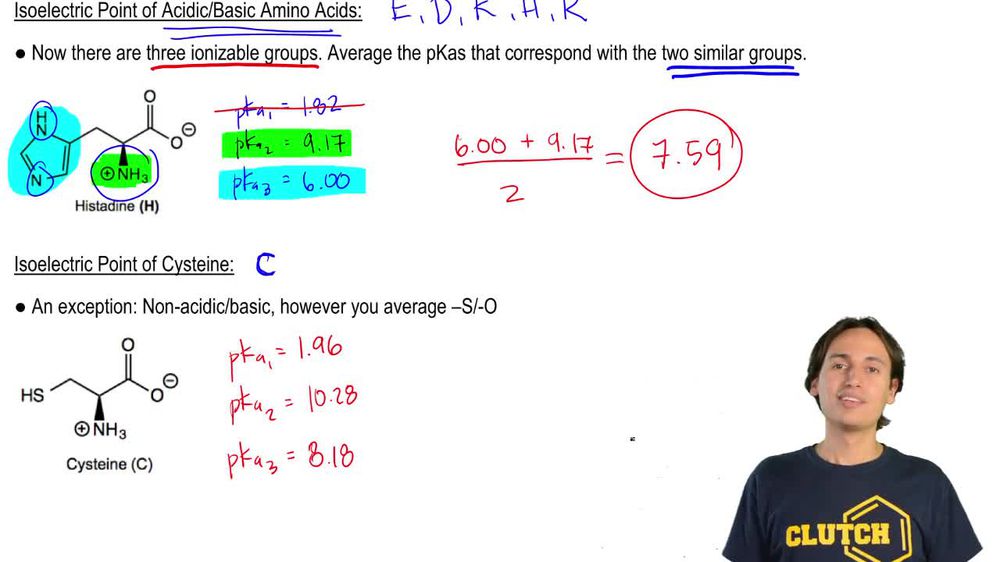
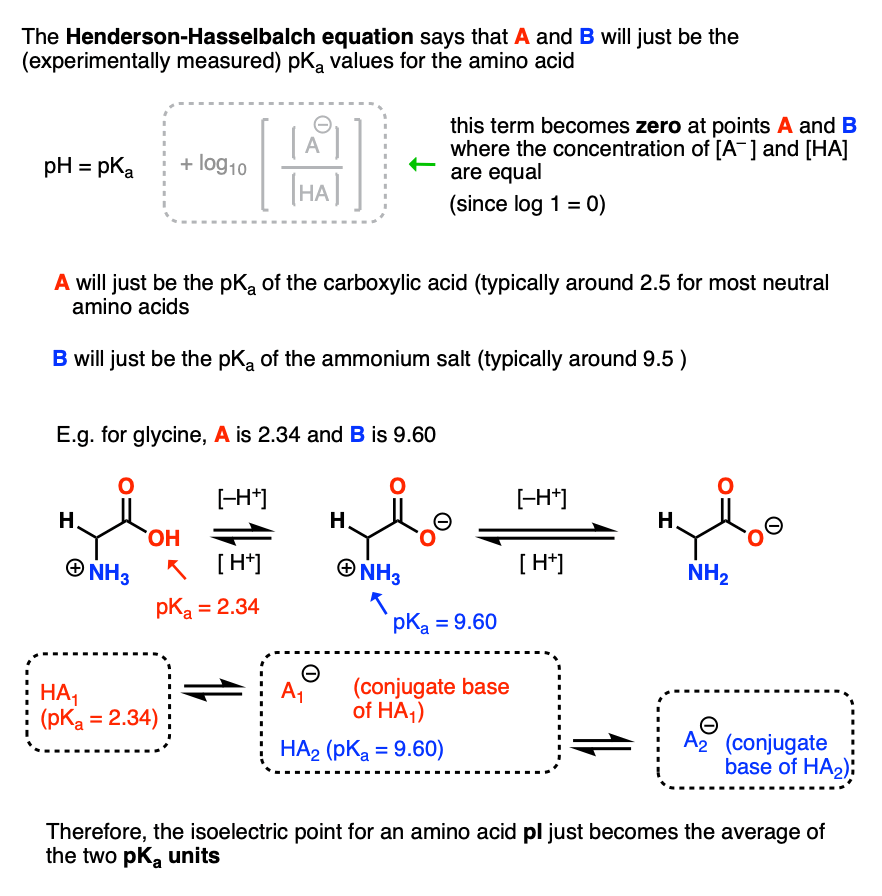
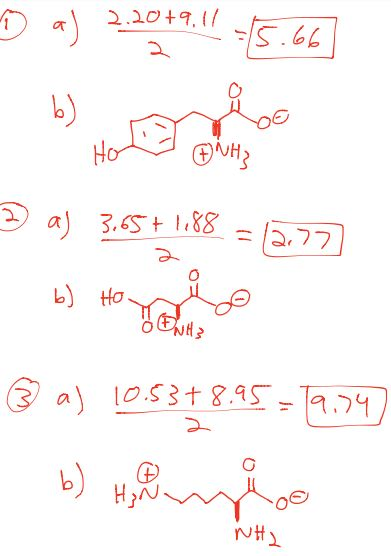
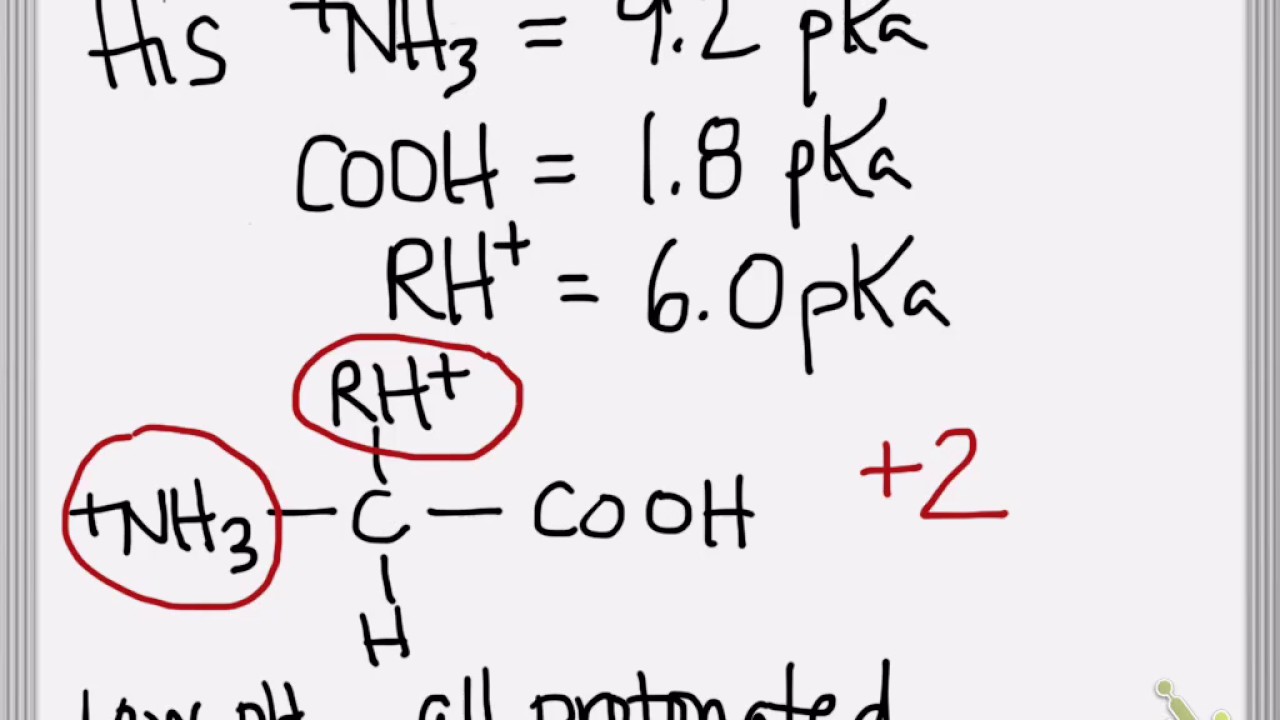
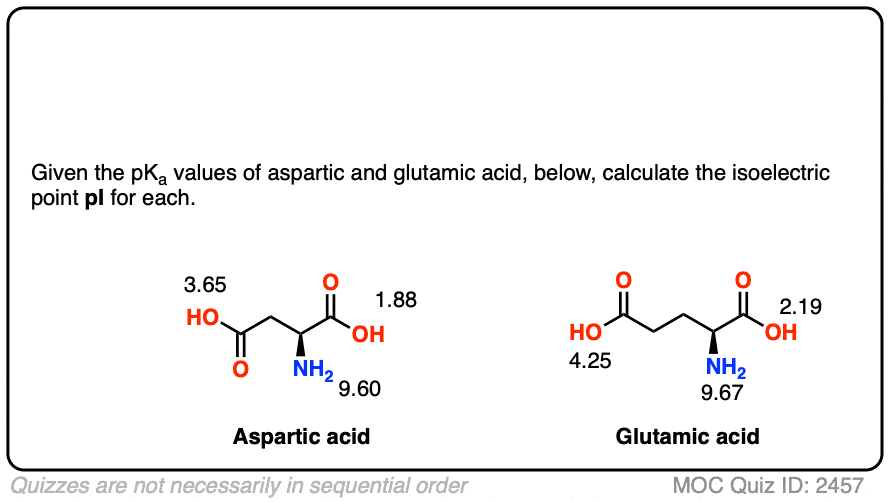
HOOC - ^⊕NH3 |CH - CH2COOH ) (A)The structure of aspartic acid is given.The pKa1,pKa2 and pKa3 of (A) respectively are: 1.88,3.65 and 9.60 . pKa1 corresponds to the ionisation of
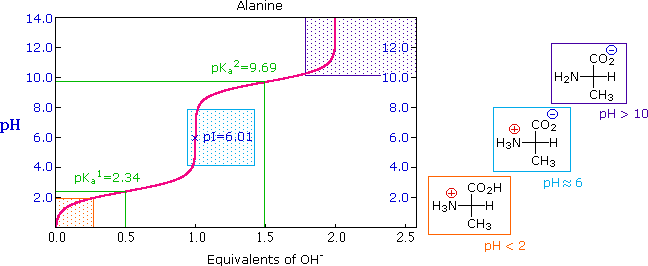
26.3: Amino Acids, the Henderson-Hasselbalch Equation, and Isoelectric Points - Chemistry LibreTexts

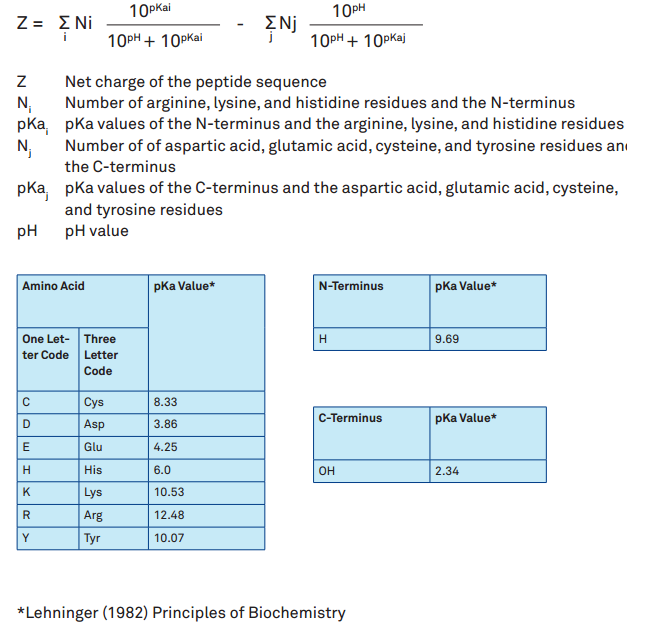
biochemistry - How can I properly calculate the isoelectric point (pI) of amino acids? - Chemistry Stack Exchange

pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides | Journal of Chemical Information and Modeling